Abstract
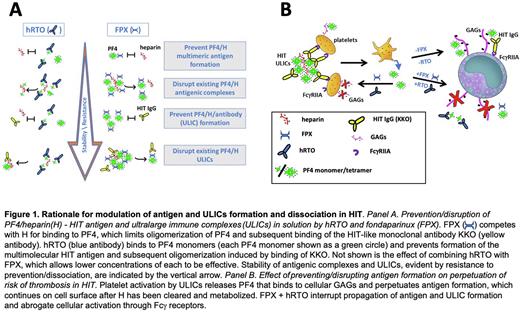
Heparin-induced thrombocytopenia (HIT) is serious prothrombotic disorder in which the target antigen, composed of tetramers of platelet factor 4 (PF4) and heparin or cellular glycosaminoglycans (GAGs), forms ultralarge immune complexes (ULICs) with a subset of anti-PF4 antibodies. ULICs initiate prothrombotic responses in large part by engaging Fcγ receptors on platelets, neutrophils, and monocytes. Contemporary anti-thrombotic therapy for HIT is neither entirely safe nor entirely successful, and acts downstream of ULIC formation and Fcγ receptor-initiated generation of thrombin. Therefore, we asked whether HIT antigen and ULIC formation and stability could be modified to attenuate cellular activation by inhibiting PF4-heparin interactions with fondaparinux in conjunction with disrupting PF4 tetramers with a humanized form of a monoclonal anti-PF4 monomer antibody (hRTO, Figure 1).
Using dynamic light scattering to measure HIT antigen (PF4 10 µg/ml and heparin 0.2U/ml) and ULIC (PF4 10 µg/ml, heparin 0.2U/ml, KKO 35µg/ml) size, we found that either fondaparinux (FPX 5-20U/ml) or hRTO (400 - 1000 µg/ml) inhibited formation of HIT antigen and ULIC, and promoted HIT antigen and ULIC disassembly. Successively higher concentrations of hRTO were required as PF4 formed large antigenic complexes and then ULICs, likely because the bonds between PF4 and UFH have been strengthened and access to inhibitors lessened (Figure 1A). Combination of fondaparinux and hRTO was effective below the concentration of either alone (FPX 2.5U/ml + hRTO 330 µg/ml). Importantly, fondaparinux and hRTO blocked Fcγ receptor-dependent induction of factor Xa activity by monocytic THP1 cells and activation of human platelets in whole blood (Figure 1B). When combined with hRTO, fondaparinux inhibited HIT antigen and immune complex formation at concentrations at or below those used clinically to inhibit FXa coagulant activity. Even lower concentrations of inhibitors (FPX < 0.1-0.5 U/ml + hRTO 50 µg/ml) were required to block cell activation than were required to act on ULICs in solution, likely because the affinity of PF4 for GAGs is less than for UFH.
These studies show that HIT antigen and immune complexes are dynamic and amenable to modulation. Fondaparinux can be converted from an anticoagulant that acts at a downstream amplification step into a rational, disease-specific intervention that blocks ULIC formation when used in combination with hRTO. Our studies demonstrate that interventions that prevent ULIC formation and stability might increase the efficacy, permit use of lower doses, and shorten the duration of anti-thrombotic therapy and help prevent this serious thrombotic disorder.
Disclosures
Rauova:Astra Zeneca: Research Funding; Rigel Pharmaceuticals Inc: Research Funding. Cai:Boehringer Ingelheim Pharmaceuticals, Inc: Current Employment. Arepally:Annexon Biosciences: Research Funding; Alexion Pharmaceuticals: Research Funding; AstraZeneca: Consultancy; Veralox: Consultancy; Biokit: Patents & Royalties. Khandelwal:BTG International Inc.: Research Funding; Annexon Biosciences: Research Funding; Alexion Pharmaceuticals: Research Funding. Poncz:AstraZeneca: Research Funding; Johnson n Johnson: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal